- Author Curtis Blomfield blomfield@medicinehelpful.com.
- Public 2024-01-02 05:35.
- Last modified 2025-01-23 17:01.
Zinc is an important element for the human body. Its deficiency threatens the development of diseases of the thyroid gland, liver, disorders of the nervous system. A person receives the substance along with food in combination with other microelements. The element is widely used in industry and if safety precautions are not followed, zinc poisoning occurs. The symptoms of intoxication are specific, at the first manifestations you should seek help.
What is zinc

Zinc is a silvery metal, number 30 in the periodic table of Mendeleev. Pure zinc does not occur in nature, only in combination with other chemical elements. The radioactive metal is zinc s alts.
In the earth's crust, the metal is found in the composition of sulfide ores and minerals. In its pure form, zinc is a dull silvery color, with zincite, willemite, sulfide and other minerals giving it various shades.
For the first time metal without impurities was obtained in the 16th century. Since then, it has been actively used in medicine, pharmacology,industry. Widespread has caused zinc poisoning. Under the influence of high temperatures, small particles of the element are released into the atmosphere. If safety measures are not followed, vapors and dust enter the human body, causing toxic effects.
Zinc in the human body
Zincum, Zn is necessary for the normal functioning of the body. Its role in the body is difficult to overestimate:
- part of carbonic anhydrase - a substance involved in the formation of hydrochloric acid;
- participates in the transport of carbon dioxide, transfers bicarbonates from tissue capillaries with blood to the lungs;
- maintains the acid-base balance in the blood;
- stimulates pituitary tropic hormones that regulate the functions of the endocrine glands;
- regulates the production and biological action of insulin;
- participates in the metabolic processes of lipids and cholesterol, normalizing fat metabolism, accelerates the process of lipid breakdown;
- prevents fatty liver disease;
- regulates the functions of seminal vesicles and exocrine tubular alveolar gland
The human body contains approximately 2-3 g of zinc. Deficiency or excess leads to disruption of the synthesis of metalloproteins. The normal requirement for an adult in zinc is 10-15 mg per day.
Hazardous zinc compounds

The industry uses "pure" zinc in compounds.
- Zinc oxide (ZnO) is widely used in industry. It is used in the manufacture of rubberdental cement, cosmetics. In the process of melting, zinc oxide emits a fine aerosol. Vapors are toxic when inhaled.
- Zinc Phosphide (Zn3P2) is used as a rodent control method. The toxic substance interacts well with hydrochloric acid, which is part of the gastric juice. The poison is effective in developing resistance to other poisons in rats and mice. In humans, zinc phosphide poisoning occurs when large amounts of fumes are inhaled.
- Zinc chloride (ZnCl2) is used in the pulp and paper industry, tinning, soldering. Causes chemical burns on contact with skin.
- Zinc sulfate is used in agriculture as a fertilizer. It is in demand in pharmacology, eye drops based on it are used for conjunctivitis, blepharitis. Zinc sulfate is a food additive for farm and domestic animals. In humans, it causes intoxication when the concentration in the atmosphere exceeds 5 mg / m³. causes ulcers if it comes into contact with the skin.
Symptoms of acute and chronic intoxication

Zinc toxicity can be acute or chronic. The first usually occurs during metal heating processes. In acute zinc poisoning, symptoms appear immediately:
- sweet taste in mouth;
- loss of smell without stuffy nose;
- within an hour or two there is a strong thirst, because the metal particles damage the receptors of the mucous membranes, the personhe doesn't seem to be drunk;
- getting into the trachea, dust causes bouts of choking cough;
- painful chest tightness, difficulty breathing;
- nausea, severe vomiting.
Chronic poisoning is much more dangerous. The metal enters the body in small doses and settles mainly in the liver and kidneys. Symptoms do not appear immediately, the person does not even realize that they are caused by the toxic effects of the metal. Signs of chronic poisoning:
- nausea in the morning;
- pain in the abdomen, epigastrium, lower back;
- regular bowel disorder;
- after exercise there are cramps in the calf muscles;
- loss of appetite;
- shortness of breath when walking fast;
- tinnitus;
- drowsiness, fatigue.
People who often come into contact with metal must follow safety precautions. Seek immediate medical attention if any symptoms appear.
Symptoms of zinc poisoning from welding

Zincum is composed of five isotopes. Also known are 15 radioactive nuclei of a chemical element. Zinc interacts well with many metals. Reacts well with acids, alkalis, ammonium s alts, molecular chromium and bromine. This far from complete list of physical and chemical properties allows the substance to be used in various branches of human activity.
Zinc poisoning in most cases occurs at industrial facilities. There is no pure metal in nature, it is obtained byexposure to high temperatures and various chemical compounds. In the process of melting (for example, when welding pipes), zinc oxide will release vapors and a fine aerosol.
Particles enter the body through inhalation and ingestion. The metal irritates the mucous membranes. Settling on the walls of the organs of the upper and lower respiratory tract, it causes coughing, inflammation of the bronchi and lungs. In severe cases, holes may form in the plate separating the nasal passages. When ingested, it causes dyspeptic disorders - nausea, vomiting, diarrhea.
Zinc dust tends to settle on the skin, causing ulceration, especially on the back of the hands.
Consequences of toxic exposure to zinc vapor

Zinc reacts well with acids contained in human biological fluids. Zinc is poorly excreted from the body, with constant contact it quickly accumulates, which contributes to the development of complications. In chronic poisoning with zinc vapor, atrophic changes in the mucous membranes develop. The consequences are manifested in the form of severe diseases:
- hypochromic anemia (hemoglobin concentration in red blood cells is less than 30 picograms);
- progressive pneumoconiosis (fibrosis of lung tissue);
- impaired ventilation and circulation of the lungs (emphysema);
- pulmonary edema;
- toxic pneumonia;
- small-spotted dissemination;
- increased urobilin in urine;
- erosive lesion of the mucosa of the bulbar small intestine (erosive bulbitis);
- gastric ulcer.
First aid
Excess zinc is perceived by the body as a poison, the first signs are the same as with any other poisoning. Each organism is individual, in the presence of chronic diseases, the consequences of intoxication can be unpredictable. A person needs help, but later (and as soon as possible), he must definitely see a doctor.
Zinc poisoning occurs most often during welding. At enterprises, next to the safety instructions, there is a memo with information on the procedure for providing first aid:
- Evacuation of the victim from the affected area, interruption of contact with the toxic substance.
- Providing fresh air: stretch the buttons near the throat, loosen the belt on the trousers;
- Providing plenty of drink.
- In case of zinc phosphide poisoning, a weak solution of potassium permanganate (0.1%) is given.
- In case of intoxication with zinc chloride, I wash the stomach by artificial vomiting.
When to Seek Medical Care

Zinc poisoning is a severe intoxication. If high doses of metal enter the body, it is necessary to be examined. Hospitalization is required under the following conditions:
- when providing independent first aid, the condition of the victim only worsens;
- a person vomits continuously, blood impurities are observed in the masses;
- skin turns pale, fingers and toes become cold;
- unconditional hospitalization is subject to small children, pregnant women, elderly people;
- the victim rolls his eyes, there is a coma.
As a rule, the company always has a full-time medical officer who is able to provide qualified first aid. If you call an ambulance at the first manifestations of intoxication, you will be able to avoid serious consequences.
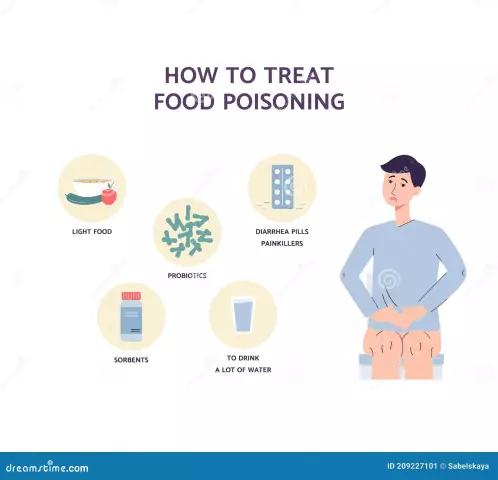
Treatment of zinc poisoning
There are no special antidotes that neutralize metal. In the hospital, general anti-toxic measures are taken to reduce the concentration of a substance in the body. They are as follows:
- Gastric lavage. The procedure is carried out using a gastric tube, introducing a solution of sodium bicarbonate (3%) through it.
- Using detoxifying agents. The victim is injected intramuscularly with 5-10 ml of Unithiol solution.
- Restoring carbohydrate balance. Intravenously administered glucose solution with ascorbic acid.
Symptomatic treatment of zinc poisoning during welding is also carried out:
- eliminate the gag reflex;
- stool normalization;
- for skin burns, local anesthetics and regenerating agents are used.
The patient undergoes a full examination, and if diseases caused by the toxic effects of the metal are detected, appropriate therapy is prescribed.
Prevention of poisoning

Zinc compounds in excess concentrations pose a threat tohuman he alth. Preventive measures should be taken to avoid intoxication:
- The process of melting non-ferrous metals containing zinc must be mechanized.
- The work area should have good general ventilation.
- Respirators, industrial gas masks and other protective equipment should be used during the work process.
- Before work, hands are treated with a greasy cream, then washed with an alkaline solution.